Featured Publications
Researchers used spatial proteomics data generated on MIBIscope to investigate tissue samples from Diffuse large B-cell lymphomas, characterize cellular organization, and classify samples based on tumor-immune microenvironment features.
Diffuse large B-cell lymphomas have spatially defined, tumor-immune microenvironments revealed by high-parameter imaging
Wright, et al., Blood Adv 14 Aug 2023
Key Insights
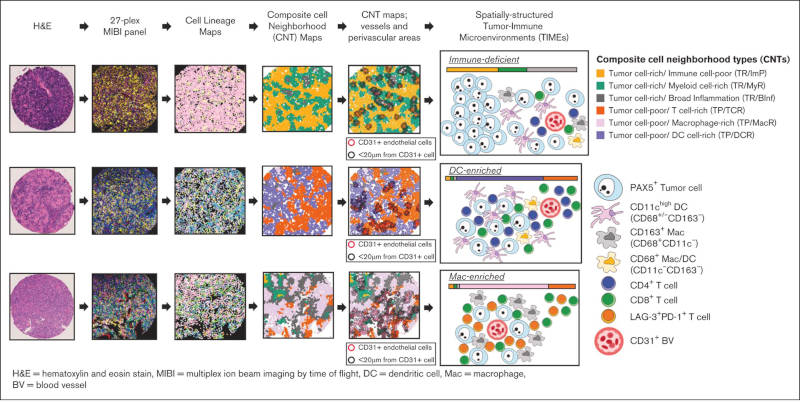
- The authors identified 6 different types of cell neighborhoods in Diffuse Large B-Cell Lymphoma Not Otherwise Specified (DLBCL-NOS) tumor-immune microenvironment (TIME) that modeled the composition of local tumor and immune cells.
- Patient sample classification based on aggregate abundance of identified TIME cell neighborhoods revealed 3 consensus DLBCL tumor categories; immune-deficient, dendritic cell–enriched (DC-enriched), and macrophage-enriched (Mac-enriched).
- Using highly-multiplexed spatially resolved protein analysis the authors characterize the TIME and describe 3 categories of DLBCL-NOS microenvironments with distinct patterns of tumor and immune cell localization and proximity to blood vessels.
Abstract
Diffuse large B-cell lymphoma (DLBCL) not otherwise specified is the most common aggressive non-Hodgkin lymphoma and a biologically heterogeneous disease. Despite the development of effective immunotherapies, the organization of the DLBCL tumor-immune microenvironment (TIME) remains poorly understood. We interrogated the intact TIME of 51 de novo DLBCLs with triplicate sampling to characterize 337 995 tumor and immune cells using a 27-plex antibody panel that captured cell lineage, architectural, and functional markers. We spatially assigned individual cells, identified local cell neighborhoods, and established their topographical organization in situ. We found that the organization of local tumor and immune cells can be modeled by 6 composite cell neighborhood types (CNTs). Differential CNT representation divided cases into 3 aggregate TIME categories: immune-deficient, dendritic cell–enriched (DC-enriched), and macrophage-enriched (Mac-enriched). Cases with immune-deficient TIMEs have tumor cell–rich CNTs, in which the few infiltrating immune cells are enriched near CD31+ vessels, in keeping with limited immune activity. Cases with DC-enriched TIMEs selectively include tumor cell–poor/immune cell–rich CNTs with high numbers of CD11c+ DCs and antigen-experienced T cells also enriched near CD31+ vessels, in keeping with increased immune activity. Cases with Mac-enriched TIMEs selectively include tumor cell–poor/immune cell–rich CNTs with high numbers of CD163+ macrophages and CD8 T cells throughout the microenvironment, accompanied by increased IDO-1 and LAG-3 and decreased HLA-DR expression and genetic signatures in keeping with immune evasion. Our findings reveal that the heterogenous cellular components of DLBCL are not randomly distributed but organized into CNTs that define aggregate TIMEs with distinct cellular, spatial, and functional features.