Featured Publications
A Stanford team used MIBI-based spatial proteomics to understand the inflammatory state of immunotherapy-driven gastritis
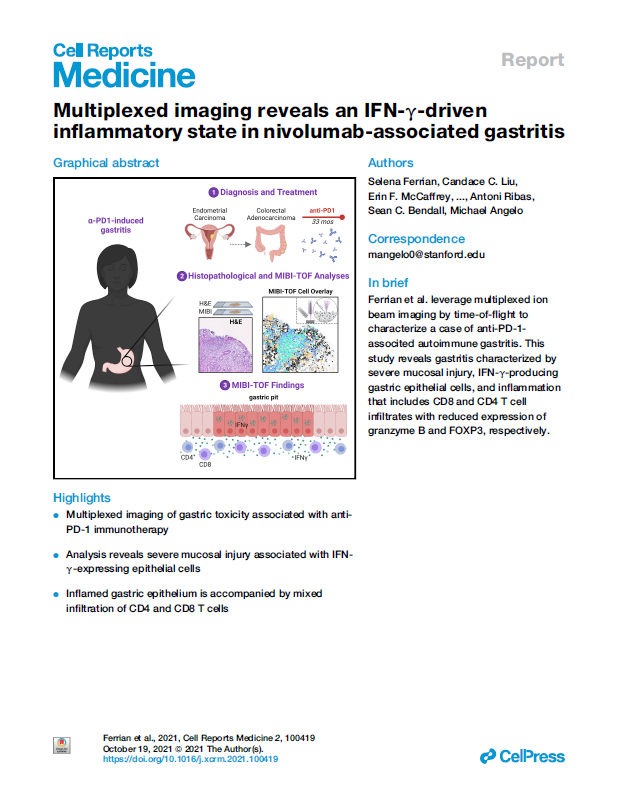
Multiplexed imaging reveals an IFN-γ-driven inflammatory state in nivolumab-associated gastritis
Ferrian, et al., Cell Reports Medicine 19 Oct 2021, 2(10), 100419.
Key Insights
- Scientists from Stanford University and the University of California, Los Angeles, performed an investigation of gastrointestinal side effects from immune checkpoint PD-1 cancer therapy based on a patient with Lynch syndrome and eight healthy controls.
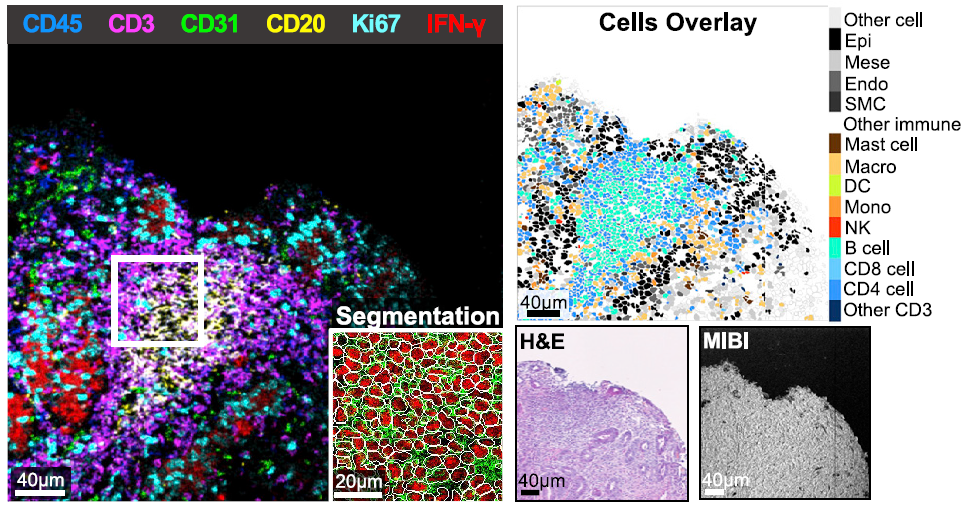
- They used MIBI™ technology to image gastric tissue samples and analyze 28 markers, revealing key molecular drivers of the inflammatory state.
- These discoveries will fuel future studies of immunotherapy-induced inflammation in the GI tract to inform better treatment strategies for patients with cancer and related conditions.
Abstract
Immune checkpoint blockade using PD-1 inhibition is an effective approach for treating a wide variety of cancer subtypes. While lower gastrointestinal (GI) side effects are more common, upper gastrointestinal adverse events are rarely reported. Here, we present a case of nivolumab-associated autoimmune gastritis. To elucidate the immunology underlying this condition, we leverage multiplexed ion beam imaging by time-of-flight (MIBI-TOF) to identify the presence and proportion of infiltrating immune cells from a single section of biopsy specimen. Using MIBI-TOF, we analyze formalin-fixed, paraffin-embedded human gastric tissue with 28 labels simultaneously. Our analyses reveal a gastritis characterized by severe mucosal injury, interferon gamma (IFN-γ)-producing gastric epithelial cells, and mixed inflammation that includes CD8 and CD4 T cell infiltrates with reduced expression of granzyme B and FOXP3, respectively. Here, we provide a comprehensive multiplexed histopathological mapping of gastric tissue, which identifies IFN-γ-producing epithelial cells as possible contributors to the nivolumab-associated gastritis.